Abstract
Background:Differentiating treatment-induced injury from recurrent high-grade glioma is an ongoing challenge in neuro-oncology in part due to lesion heterogeneity.This study aimed to determine whether different MR features were relevant for distinguishing recurrent tumor from the effects of treatment in contrast-enhancing(CEL)and non-enhancing lesions(NEL).
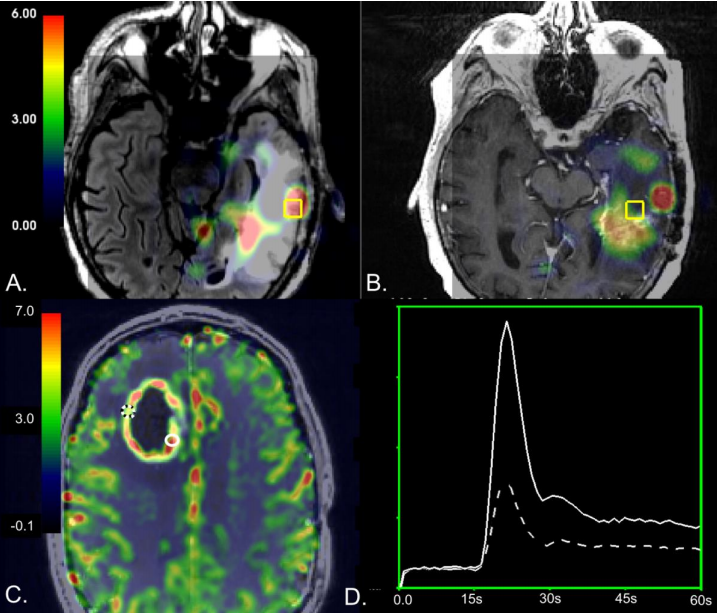
Introduction:Tumor recurrence in patients with high-grade glioma(HGG)is difficult to diagnose because treatment-induced injury often appears identical on conventional anatomic magnetic resonance(MR)imaging.It is estimated that 25 to 35 percent of patients who undergo standard of care radiation and chemotherapy in the form temozolomide for HGG experience treatment-related injury,1–3 and its appearance is even more common with the recent advent of immuno-and other targeted therapies in clinical trials.If recurrence is incorrectly diagnosed,a patient may be removed from an effective therapy,which could invalidate the results of a clinical trial or expose a patient to unnecessary surgical intervention.To mitigate these risks,identifying the exact location and extent of treatment related changes within newly enlarging lesions is critical.To overcome the complications introduced by tissue heterogeneity in ROI-based studies,one strategy is to use image-guided tissue samples of known coordinates to directly map MRI characteristics to histopathology.In 2002,Rock et al.pioneered this technique in distinguishing radiation necrosis from recurrent disease using metabolite ratios derived from 1H MR spectroscopic Imaging(MRSI)at the location of sampled tissue17.Their findings suggested that the ratios of choline and lactate/lipid to creatine could differentiate samples with pure necrosis and tumor,but not those with mixed pathology.In 2009,Hu et al.utilized this technique in conjunction with Dynamic Susceptibility Contrast(DSC)perfusion-weighted imaging to distinguish post-treatment radiation effect from recurrent tumor with high sensitivity and specificity using relative cerebral blood volume(rCBV)values from 13 patients.The goal of this study was to determine whether different MR characteristics were relevant for distinguishing pathological features of recurrent tumor from the effects of treatment in the contrast enhancing and non-enhancing lesions of recurrent high-grade gliomas by leveraging a unique dataset of image-guided tissue samples of known coordinates to avoid complications of tissue heterogeneity that confounds most lesion-level analyses.Based on prior literature,we expect that samples comprised of recurrent tumor will have increased blood volume and abnormal metabolism,with decreased diffusion compared to samples containing treatment-effect.We also hypothesize that:1)this difference would be more pronounced in diffusion and perfusion metrics for samples within the contrast enhancing lesion(CEL),while metabolic measures would be equally effective at differentiating recurrent tumor from treatment effect in both the contrast enhancing and nonenhancing lesions(NEL);and 2)the addition of multiparametric physiologic and metabolic MRI in conjunction with tissue sample level analyses will provide increased sensitivity and specificity in distinguishing recurrent tumor from the effects of treatment in both types of lesions compared to anatomical imaging.
Methods:This prospective study analyzed 291 tissue samples(222 recurrent tumor;69 treatment-effect)with known coordinates on imaging from 139 patients that underwent preoperative 3T MRI and surgery for a suspected recurrence.8 MR parameter values from perfusion-weighted,diffusion-weighted,and MR spectroscopic imaging at each tissue sample location were tested for association with histopathological outcome using generalized estimating equation models for CEL and NEL tissue samples.Individual cutoff values were evaluated using ROC-Curve analysis with 5-fold cross-validation.
Results:In tissue samples obtained from the CEL,elevated relative cerebral blood volume(rCBV)was associated with the presence of recurrent tumor pathology(p<0.03),while increases in normalized choline(nCho)and choline-to-NAA index(CNI)were associated with the presence of recurrent tumor pathology in NEL tissue samples(p<0.008).A mean CNI cutoff value of 2.7 had the highest performance,resulting in mean sensitivity and specificity of 0.61 and 0.81 for distinguishing treatment-effect from recurrent tumor within the NEL.
Conclusion:Although our results support prior work that underscores the utility of rCBV in distinguishing the effects of treatment from recurrent tumor within the contrast enhancing lesion,we found that metabolic parameters may be better at differentiating recurrent tumor from treatment-related changes in the NEL of high-grade gliomas.

背景:区分治疗性损伤和复发性高级别胶质瘤是神经肿瘤学的一个持续挑战,部分原因是病变的异质性。本研究的目的是确定不同的磁共振特征是否有助于区分复发肿瘤与对比增强(CEL)和非增强病变(NEL)的治疗效果。
简介:高级别胶质瘤(HGG)患者的肿瘤复发很难诊断,因为治疗引起的损伤在常规的解剖磁共振(MR)成像上往往是相同的。据估计,接受替莫唑胺治疗的HGG患者中有25%至35%的患者经历了治疗相关的损伤。随着较近临床试验中免疫疗法和其他靶向治疗的出现,这种情况更为常见。如果复发被错误诊断,患者可能会被排除在合适治疗之外,这可能会使临床试验的结果无效,或使患者面临不必要的外科干预。为了减轻这些风险,确定新扩大病灶内治疗相关变化的确切位置和范围是至关重要的。在基于ROI的研究中,为了克服组织异质性带来的并发症,一种策略是使用已知坐标的图像引导组织样本直接将MRI特征映射到组织病理学。这项技术提出了利用1H磁共振波谱成像(MRSI)在取样组织位置获得的代谢物比率来区分放射性坏死和复发性疾病。他们的研究结果表明,胆碱、乳酸/脂质与肌酸的比值可以区分单纯坏死和肿瘤,但不能区分混合病理。将此技术与动态敏感度对比(DSC)灌注加权成像相结合,利用13例患者的相对脑血容量(rCBV)值,以高灵敏度和特异性区分治疗后放疗效应和复发性肿瘤。本研究的目的是通过利用一组已知坐标的图像引导组织样本的独特数据集,确定不同的MR特征是否与区分复发性高级别胶质瘤的对比度增强和非增强病变的治疗效果相关为了避免组织异质性的并发症,而这些并发症会干扰大多数病变水平的分析。基于先前的文献,我们预计与含有治疗效果的样本相比,由复发肿瘤组成的样本会有血容量增加和代谢异常,扩散减少。我们还假设:1)对比增强病灶(CEL)内样本的扩散和灌注指标的差异将更为,而代谢测量在区分增强和非增强病变(NEL)的复发肿瘤和治疗效果方面同样合适;2)与解剖成像相比,多参数生理学和代谢MRI结合组织样本水平分析将提高区分复发性肿瘤和治疗效果的敏感性和特异性。
方法:本研究分析了139例疑似复发患者术前3tmri和手术治疗的291例已知坐标的组织样本(222例复发肿瘤;69例治疗效果)。利用CEL和NEL组织样本的广义估计方程模型,测试了每个组织样本位置的灌注加权、扩散加权和MR光谱成像的8个MR参数值与组织病理学结果的相关性。使用ROC曲线分析和5倍交叉验证来评估个体的临界值。
结论:虽然我们的研究结果支持了先前的研究,强调了rCBV在区分造影增强病灶内治疗效果和复发肿瘤方面的作用,但我们发现代谢参数在区分高级别胶质瘤的复发性肿瘤和治疗相关的变化方面可能更好。
关于美国Mitchel Berger教授

Mitchel Berger教授来自美国神经外科医院三、国际闻名的神经外科疾病研究和治疗机构UCSF医学中心。Mitchel Berger教授是全美公认的成人及儿童脑肿瘤治疗专家,在任职UCSF神经外科主席之前,他还担任过美国神经外科协会主席、美国神经学医师协会主席、美国癌症研究协会州立法委员会主席、北太平洋神经及神经外科和精神病学会主席,在神经外科疾病临床咨询、癌症研究和基因治疗等方面有突出贡献。目前,Mitchel Berger教授是UCSF医学中心SPORE脑肿瘤项目的研究员,也是美国国家“癌症登月”计划蓝带小组专家。
除了在美国享誉盛名,Mitchel Berger教授更是神经外科领域内清醒开颅手术、脑功能监测技术方面的专家,在脑部术中建图方面拥有丰富的知识,能够准确识别运动、感觉和语言功能的部位,从而避免在手术过程中伤及重要神经,能在很大水平上保障手术的准确性和顺利性。由Mitchel Berger教授发明的超声引导手术器械方法获得了美国专利商标局(USPTO)授予的专利,目前已在神经外科领域广泛推广使用。
原文链接:https://www.sci-hub.pl/10.1093/neuonc/noaa094




